Weaner Pain Relief Trial – Producer Demonstration Sites WA
Can pain relief husbandry practices improve animal health and well-being?
In alignment with the Australian Beef Sustainability Framework’s objective of achieving 100% utilisation of pain relief for husbandry practices by 2030, the Weaner Pain Relief Trial was launched to evaluate the effectiveness of both short and long-acting pain relief products within the context of large-scale northern beef enterprises. This initiative, prompted by producer demand, sought not only to improve animal health and well-being but also to educate producers on the advantages of integrating pain relief into standard husbandry procedures.
What constitutes genuine pain relief?
In addition to administering injections, genuine pain relief involves:
- Attending to the animals’ needs
- Conducting husbandry procedures at an early age
- Maintaining high standards of hygiene and providing suitable facilities
- Employing appropriate weaning techniques
- Ensuring proper nutrition
- Observing and caring for animals’ post-treatment
- Effectively managing wounds
Integrated R&D Pain Relief PDS
This project, a collaborative effort between Western Australia, the Northern Territory, and Queensland state governments, with co-investments from Meat and Livestock Australia (MLA), focused on elevating the efficacy of Meloxicam and Tri-solfen® in improving animal welfare during castration and dehorning. The research was carried out across three commercial Producer Demonstration Sites (PDS) located in the Kimberley and Pilbara regions. A total of 1,619 Bos indicus, and Bos indicus cross, weaners were assessed for physiological and behavioural changes following these procedures.
Trial Design
The pain relief trial consisted of two methods, a 35-day and 21-day model as represented in Figures 1 and 2. The 35-day trial model was the more intensive of the two methods and recorded baseline data prior to weaner processing. Unlike the 21-day model, this trial allocated animals to their respective treatment groups, recorded initial live weight (LW), and fitted accelerometer devices on day 1 (a). The weaners were then turned out into their weaner paddocks where baseline data was recorded on accelerometer devices. After two weeks, the trial animals returned to the yards for processing (branded, tagged, and vaccinated)(b). Their LWs were recorded, and accelerometers removed and replaced with fully charged devices. After all crush side data was recorded, weaners returned to their paddocks for a further three weeks before returning to the yards. On day 35 of the trial (21 days post-procedure) animals returned to the yards for final assessment (c). LWs were recorded, castration and dehorning sites were assessed, and accelerometer devices were removed for analysis.
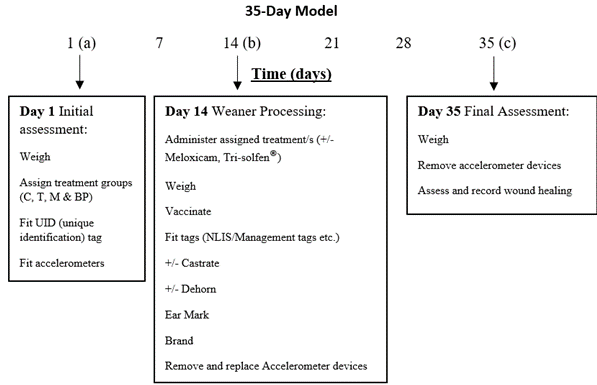
Figure 1. The 35-day trial model.
The 21-day model differed from the 35-day trial by recording LW and fitting GPS technology and accelerometers on day 1 of the trial coinciding with weaner processing (a). Trial animals returned to the yards 21 days later where GPS collars and accelerometers were removed, and final assessments completed (b). This model was designed as a short, practical trial model, which only required weaners to be mustered twice over the study period. This model reflected typical station practices and fitted in with mustering programs.
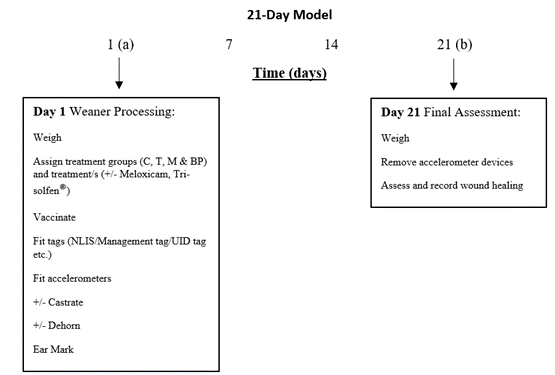
Figure 2. 21-Day trial model.
Treatment groups and allocations
Weaners were randomly assigned to one of four treatment groups:
- Control (C)
- Tri-solfen® (T)
- Meloxicam (M), and a
- multimodal Meloxicam and Tri-solfen® (MT)
The Control group did not receive any pain relief during processing, while the other treatment groups received either one or both pain relief products. Tri-solfen®, a topical local anaesthetic, was sprayed on the dehorning and surgical castration sites post-procedure, while Meloxicam (Metacam), a nonsteroidal anti-inflammatory drug (NSAID), was administered via injection 3-5 minutes prior to processing.

Figure 3. Injectable NSAID Meloxicam and topical Tri-solfen ® spray
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID). It works by reducing hormones that cause inflammation and pain in the body. It can be given through an injection or orally prior to the procedure and provides pain relief for 26-72 hours.
Tri-solfen ® is a unique post-operative drug sprayed onto the animal post-procedure. The active ingredients include Lignocaine (fast-acting anaesthetic), Bupivacaine (long-acting anaesthetic), Adrenaline (constricts blood vessels) and Cetrimide (antiseptic). The local anaesthetic blocks sensory nerves which cause pain and provides relief from pain for approximately 24 hours.
Method
Over two consecutive seasons, three Western Australian properties each provided 400-600 weaner animals for the trial. These animals were randomly distributed between the four treatment groups. Behavioural observations were made during weaner processing, and GoPro cameras captured post-processing behaviours. After approximately 21 days, the weaner animals returned to the yards for weighing and an inspection of their castration and/or dehorning. Some weaner were also fitted with GPS collars and accelerometer devices to monitor changes in animal behaviour among the treatment groups.

Figure 4. DPIRD beef development officer Sarah Gwynne fitting a GPS collar to a weaner steer
Preliminary Findings
The trial highlighted the significant impact of castration and dehorning on animal health and well-being. It is evident that these procedures negatively affect weight gain when compared to non-castrated and horned animals. Importantly, the provision of pain relief did not mitigate this impact. This trial emphasizes the vital importance of best practice animal welfare, including conducting husbandry procedures at an early age, maintaining high standards of hygiene, providing suitable facilities, ensuring appropriate weaning techniques and observing and caring for animals post-treatment.
The Weaner Pain Relief Trial, driven by the Australian Beef Industry’s commitment to animal welfare, provides invaluable insights into pain relief’s effects during routine husbandry procedures. By exploring the benefits and limitations of pain relief products, this research project aims to shape the future of animal husbandry practices in the Australian beef industry, ultimately enhancing the well-being of cattle and the sustainability of beef production.
This project is a collaborative effort between Western Australia, the Northern Territory, and Queensland state governments, with co-investments from Meat and Livestock Australia (MLA).